Research and development of key raw materials
Bio Technology R&D


Research and development of key raw materials
BALANCERX conducts continuous research and verification to scientifically demonstrate the efficacy and safety of natural bio-ingredients through experimental data.
We objectively validate the functionality and reliability of our ingredients using the core complex formulations we have developed, supported by test reports and experimental results obtained based on these formulations.



Myrrh Natural Biocomposition (BRX-01)


The natural bio-composition (BRX-01) has been scientifically validated for safety and functionality through component analysis and various experimental data.
Below are the results of component analysis, gene expression (qPCR), cytotoxicity, enzyme inhibition activity, and skin safety tests.
Ingredient Analysis
Quantification and mass spectrometry of indicator components
Confirmation of mass spectrometry spectra for the identification of skin-active components in the natural bio-composition.

Analysis of a distilled water-based sample using mass spectrometry revealed the most prominent peak at approximately 4300 m/z,
suggesting the presence of active ingredients within the natural bio-composition
Analysis of eight major active compounds from natural sources using LC/MS techniques.


Analysis of anti-aging and antioxidant
Proven anti-aging efficacy based on gene expression
Analysis of anti-aging and antioxidant gene expression

Changes in the expression of p53, GPX1, and NRF2 genes were observed following treatment with the natural compound mixture,
demonstrating their association with antioxidant defense and aging inhibition.
Cell Toxicity Assessment
Cell viability analysis
Extracts that do not damage skin cells

Even under high-concentration conditions exceeding the actual usage levels in cosmetics,
the cells remained healthy, confirming that the ingredient is safe and non-irritating to the skin.
Inhibitory Activity Assesment
Elastase, Tyrosinase inhibition

The herbal complex extract promotes collagen production in cells and increases PIP content.It also inhibits β-hexosaminidase,
which is related to allergic responses, and inflammatory factor IL-6. Furthermore, it inhibits elastase activity related to skin elasticity reduction, demonstrating anti-inflammatory, anti-allergic, and anti-wrinkle effects simultaneously.

Confirmation of Collagen Production Promotion and Inhibitory Effects on Allergic/Inflammatory Responses and Wrinkle Improvement
Skin Safety Assessment
Irritation, sensitization, and phototoxicity tests

Results from skin irritation tests, single-dose toxicity tests, skin sensitization tests, and NRU phototoxicity tests showed no irritation or toxicity to the skin or body. This confirms that the ingredients used in the cosmetics are safe and non-irritating.

Confirmation of Low-Irritation and Non-Toxic Ingredients through Various Safety Evaluations
Skin Safety Evaluation
Evaluation of skin patch safety and ocular mucosal irritation potential

According to the skin patch safety test and the ocular mucosal irritation test results, no irritation or adverse reactions were observed even after 24 hours of product application. Therefore, it was determined as non-irritating, and further tests confirmed non-irritation in ocular mucosal tissues as well. These findings confirm that the ingredient can be safely used even around the eyes.

Verification of Low-Irritation and Low-Toxicity Ingredients through Various Safety Evaluations
Test report
Antibacterial test report


Using myrrh natural bio composition (BRX-01)
Product human clinical trials
Product-based analysis results
Products formulated with the herbal complex ingredient (BRX-01) have been scientifically verified through various
experimental analyses for the stability and functionality of the ingredient.
The following results were obtained: Skin irritation test, skin patch test, ocular mucosal irritation test, moisture retention test, HFDPC proliferation evaluation, antioxidant activity evaluation, anti-inflammatory evaluation, scalp barrier evaluation, scalp soothing evaluation, and scalp keratin improvement evaluation.

Skin Irritation Test
Measurement of Skin Response

Skin Irritation Soothing Test
Redness relief

Skin Irritation Soothing Test
Skin barrier relief

Skin Irritation Relief Test
Relief of Skin Redness Caused by External Irritants

Skin Lifting Test

Compared to baseline, the skin lifting indicator showed
improvementsof –4.45% after 2 weeks and –8.08% after 4 weeks of product use.
This decrease was statistically significant (p < 0.05), confirming the lifting effect of the product.
Wrinkle Improvement Test

Compared to baseline, the wrinkle index showed
improvements of –7.44% after 2 weeks and –18.28% after 4 weeks of product use.
This reduction was statistically significant (p < 0.05), confirming the product's effectiveness in wrinkle improvement.
Melasma and Blemish Improvement Test

The melasma and blemish index showed
improvements of –1.97% after 2 weeks and –3.43% after 4 weeks of product use.
This reduction was confirmed to be statistically significant (P < 0.05).
Cooling Effect Test (Skin Temperature Reduction)

Compared to baseline, the skin temperature decreased
from 30.64 ± 1.21°C to 29.45 ± 1.28°C after product use, resulting in a –3.90% cooling effect.
This reduction was statistically significant (P < 0.05), confirming the product's efficacy in lowering skin temperature.
Skin Moisture Content Test

According to the skin moisture content test,
moisture levels increased by over 300% immediately after product use.
Even after 3 hours, the skin retained a high level of moisture,
demonstrating excellent long-lasting hydration performance.
Skin Elasticity Test

Compared to baseline, skin elasticity improved by 10.16% after 2 weeks and 18.59% after 4 weeks of product use.
This increase was confirmed to be statistically significant (p < 0.05).
Skin Soothing Test on Heat-Irritated Skin

Compared to baseline, the skin soothing index under heat-induced irritation showed
an improvement of –4.71% after product use.
This decrease was confirmed to be statistically significant (P < 0.05).
24-hour Moisture Retention Test
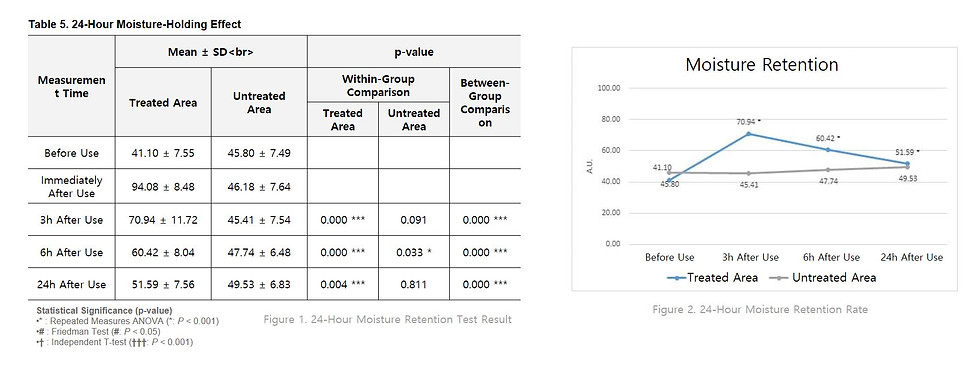
According to the measured moisture content, the treated area showed statistically significant differences compared to the untreated area at 3, 6, and 24 hours after application.
Subjective Survey Results

According to the product survey results,
users showed a very high level of satisfaction and improvement in skin condition.
91% of participants responded positively in wrinkles and overall skin condition,
and 86% expressed a desire to continue using the product.

According to the consumer survey on skin soothing and product usability,
overall satisfaction was remarkably high.
100% of respondents reported relief from skin troubles such as irritation, itching, redness, and pain caused by mask use after applying the product.
In addition, major usability attributes—including moisturization, smoothness, spreadability, and overall feel—also received 100% or higher positive response rates.

Thuja Orientalis natural bio composition (BRX-02)


The natural bio-composition (BRX-02) has been scientifically proven for its safety and functionality through component analysis and various experimental data. Below are the results of the Dermal Papilla Cell Proliferation Test and the Antioxidant Activity Evaluation.
Evaluation of HFDPC Proliferation (Hair Follicle Dermal Papilla Cell Proliferation Test)
.jpg)
As a result of applying the test to HFDPC (Human Follicle Dermal Papilla Cells), which are key cells in the hair root area responsible for hair growth,
the herbal complex extract was found to promote cell proliferation, demonstrating potential for hair root strengthening and hair loss prevention.
Antioxidant Activity Evaluation

The herbal complex extract showed antioxidant activity at high concentrations close to the raw material level.
Therefore, even when formulated in a finished product,
it is expected to deliver effects that reduce oxidative stress, which is a major cause of skin aging.

Using natural bio composition of arborvitae leaves
(BRX-02)
Product human clinical trials
Product-based analysis results
Products formulated with the Thuja Leaf Complex (BRX-02) have been scientifically validated for ingredient safety and functionality through extensive experimental analyses.
Below are the results of the Hair Elasticity Test, Scalp Soothing Evaluation, Scalp Keratin Assessment, and Consumer Survey.
Hair Elasticity Test
Clinical Test Results

The measurement of hair elasticity showed a 46.42% increase, which was statistically significant (P < 0.05).
Scalp Soothing Test
Clinical Test Results

After product use, the scalp irritation index decreased by 42.15%, demonstrating a soothing effect and improvement in scalp sensitivity. This improvement was also confirmed to be statistically significant (P < 0.05).
Scalp Keratin Test
Clinical Test Results

After product use, scalp flakiness was reduced by 29.00%.
This improvement was confirmed to be statistically significant (P < 0.05).
Survey

All participants who used the product reported experiencing 100% positive effects.
High levels of satisfaction were observed across various aspects, including improved hair elasticity, damage reduction, and scalp soothing after use.
Product composition
Product Archive
